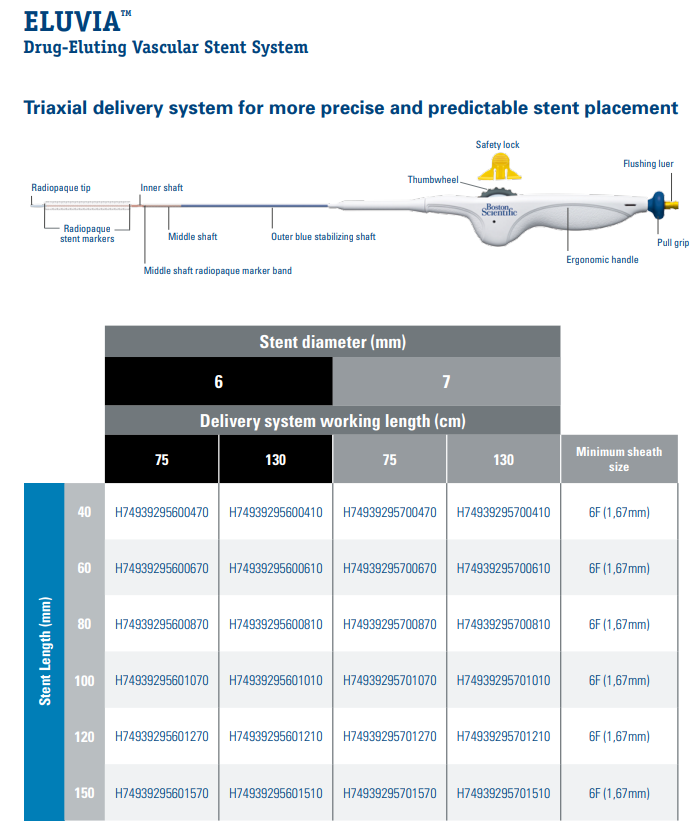
Boston Scientific, 39294-70041 Eluvia™ H74939295700410 Eluvia™ Drug-Eluting Vascular Stent System, 7 mm x 40 mm x 130 cm. Box of 01
The ELUVIA Drug-Eluting Vascular Stent System is indicated for improving luminal diameter in the treatment of symptomatic de-novo or restenotic lesions in the native superficial femoral artery (SFA) and/or proximal popliteal artery with reference vessel diameters (RVD) ranging from 4.0 - 6.0 mm and total lesion lengths up to 190 mm
- The ELUVIA Stent System consists of a self-expanding, open mesh, laser-cut nitinol stent with tantalum markers and a triaxial stent delivery system, which includes a middle sheath to protect and constrain the stent.
- The stent is loaded into the triaxial delivery system. When ready to be implanted, the stent is deployed by retracting the middle sheath of the delivery system. As the stent is exposed to body temperature, it expands to appose the vessel wall
The ELUVIA Stent System is comprised of the following components:
- Stent Component
- Stent Coating (Polymers and Drug Substance)
- Delivery Catheter
-